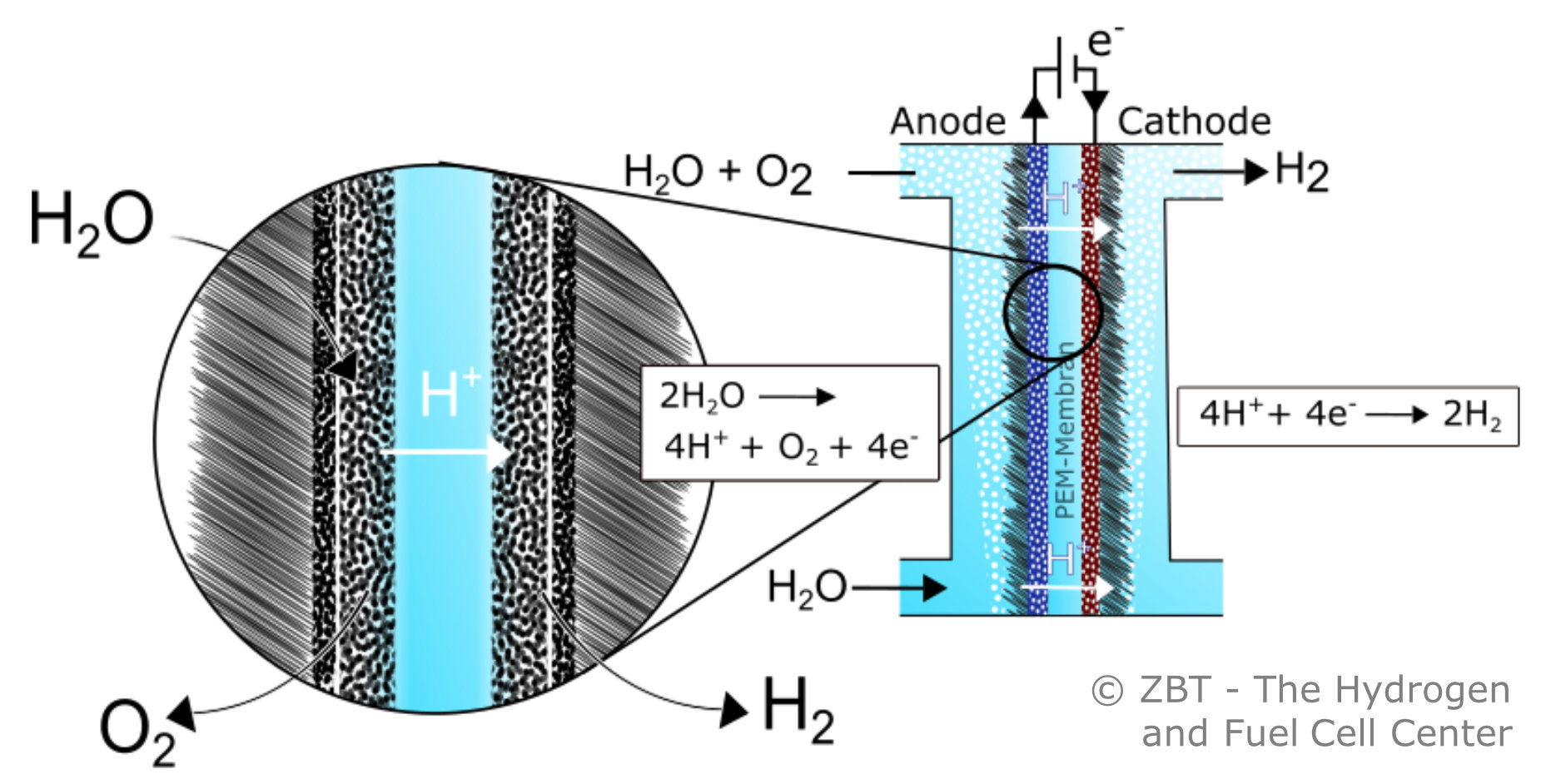
PEM water electrolysis
PEM electrolysis is one of several processes for producing hydrogen from water. The ZBT is conducting research into optimising this technology.
PEM water electrolysers are already being marketed by industry with system outputs ranging from one to several hundred megawatts. However, the advantages of PEM electrolysis, such as high efficiency, high current densities and simple system design, are accompanied by disadvantages such as the necessary use of precious materials and the resulting high investment costs.
To further improve the technology, we are therefore conducting research into materials and components as well as degradation analysis and service life prediction.
Materials and components
Since iridium catalysts are currently the main material used on the anode side for long-term operation of PEM electrolysers and these are very expensive, it is important to make the best possible use of the material in the coating. Therefore, our work focuses on the development and optimisation of catalyst coatings for the membrane.
We have a sonotrode and various rotor-stator mixers at our disposal for the production of dispersions. We can coat membranes using an ultrasonic spray process or with a squeegee (direct membrane and decal process). In addition to ex-situ analysis using SEM, XRF and confocal microscopy, we are able to carry out various electrochemical investigations on a laboratory scale up to 25 cm².

Testing and degradation analysis
Safe and predictable operation is crucial for the industrial use of electrolysers. To this end, the group operates test and degradation analysis test benches for PEM electrolysis cells and stacks and is working on the development of methods for in-situ degradation analysis. Our testing capabilities range from small electrolysis cells with a cell area of 5 cm² to test benches for stack prototypes with 500 cm² and 2000 A.
For degradation analysis, the states of the electrolyser and the process system components are continuously monitored and, based on this overall system, state analyses are performed and state predictions are made. We draw on our many years of experience in electrolysis operation and modern machine learning methods. The numerical models are supplemented with classic electrochemical methods such as polarisation curves and impedance spectroscopy.
Our goal is to be able to determine the electrolyser status based on the smallest changes in normal operation.